Good Behavioural Practices (GBP)
“At the heart of enduring success for any Pharmaceutical company lie Good Behavioural Practices- GBP”
Good Behavioural Practices - GBP emerged from our Behavioural Consulting with Pharmaceutical manufacturing Organizations. There is a business compulsion of implementing cGMP (Current Good Manufacturing Practices) in these companies if they have to get regulatory approvals for manufacturing and exporting medicines. In Indian context, getting regulatory approval from Indian authorities is an eye wash due to widespread culture of corruption. Majority of the Indian Pharma companies will miserably fail in regulatory compliances if strictly audited. Patient safety due to quality manufacturing practices does not seem to be important for Indian Pharma manufacturer. It is generalization, speaking for large majority of small scale and even medium scale set ups.
However, due to stricter auditing by International Regulatory Agencies such as WHO, US FDA, MHRA and the like, Pharmaceutical manufacturers cannot get or execute export orders unless they pass these agencies' audits. Result of this strictness is spate of disapproal of facilities of leading Indian companies like Ranbaxy, Aurobindo, RPG Life Sciences, Panacea Biotec and so on.
The real issue is that these big Pharma companies employ highly paid, technically qualified executives who fully understand cGMP but still they are failing these audits. It is not technical skill or knowledge which is lacking, it is the attitudes, mindsets, ethics and culture which is the biggest block in ethically implementing the cGMP
For instance, Validation Studies have to be conducted for various activities and processes. It is a cumbersome process which can be done through the existing competent people within the companies. But still Validation reports are made many a times without conducting studies on the ground with fake data and results. The reason can be many and it could be as simple as that it takes hard work and effort and money to do so. At times, the senior management is also implicitly involved in allowing such fake reports to be generated.
In another instance. The test results of the manufactured items are clearly showing some impurities beyond allowed limits. Discarding the batch will cost money. Quality Control (QC) department only has to make false report of tests passing, saving millions for the management. Management is happy with the QC department.
Take one more instance. GMP facility cannot be used for non GMP activities of running some experimental batch. Top Management wants it as it will save costs of getting it done from outside. The costs are saved by using the cGMP facility for non GMP batches.
The US drug regulator’s India ex-chief, Altaf Ahmed Lal says, “I want a manufacturer to ask – and be able to answer – the questions, why are we failing inspections? And what specific controls do we still need to put into place on a 24*7 basis so that on the next FDA inspection we will pass?”
Here is the clue to your answer Mr. Lal, “ Good Behavioural Practices –GBP have to be implemented before cGMP.”
Following cGMP means extra costs, botheration, rejection of batches, unhappy bosses and so on. Why do it, if one can get away by making so called small excursions? These small excursions become big excursions and then the culture takes over where GMP is only through lip service while on the ground all short cuts and costs are being saved for big profits for the Shareholders.
When confronted and asked, “Why are you doing all these violations?” The executives will tell you, “We are doing it to save costs for the management only. Isn’t it in company’s interest to save costs?”
In nutshell, no Pharma company can implement cGMP without doing extensive cultural and behavioral interventions
And this GBP starts with the top management of the company. The Head of the Organization, HOO by whatever designations (CEO, MD, JMD, Chairman, President etc) he is known has to be extensively coached on ethics. It is well known through research that ethical standards fall as we go down the hierarchy. If the standards are very high at the top, only then can we expect some level of ethical behaviour at the lower rungs.
Unfortunately, most of the HOO’s are not very evolved ethically and behaviourally. Herein lies the real issue of non implementation of cGMP. It is here that extensive work is done by behavioural experts. There have been cases where a second in command ruins the organization by saving costs through shortcuts mentioned above in the process getting accolades from the HOO. In the eyes of HOO he has done a great job of solving problems so efficiently and cost effective manner (of course in the short run, the real costs will have to be borne in the long run like Ranbaxy which paid close to US $ 500 millions in year 2013 for the sins committed in the year 2007/8). Unless the HOO has the wisdom to see through this cost effectiveness and efficiency, he will continue to be deceived by super efficient juniors.
From above we come to the second most important behavioural intervention required from GBP experts. The entire management at all levels is scanned and assessed from their behavioural competence in implementing cGMP. After assessment, those who are found good are empowered and those lacking in the competence are trained and coached or sidelined from the cGMP implementation.
The third important intervention comes by way of creating a new reward and punishment system. In the new system, those behaviours and competencies which are important in implementing cGMP are rewarded while the negative behaviours that discourage cGMP implementation are severally and unequivocally punished.
As you can understand the entire process is highly political. The power structure of the organization is being shaken and hence needs to be handled with lot of sensitivity. Needless to say that resistance to change has to be handled across all levels. This is where the real behavioural competencies of the GBP experts come to play. Change Management skills are an important part of the GBP experts’ toolkit.
There is a big picture of the entire GBP implementation which becomes clear once the GBP experts complete their diagnostics and prognastics of the organization. Unless the top management is prepared to undertake this arduous journey which may take close to eighteen months, the effort will not yield results. The GBP experts have to enjoy the full confidence of the top management along with great rapport and understanding to complete this cultural transformation successfully.
Why this trust and rapport is important will become clear from one summary case study given below.
A CASE STUDY :
Mr. Z is the HOO of the organization and Mr. Y is his second in command. The GBP experts have valid assessment through various sources and processes that Mr. Y is a fake leader who has managed to be in the good books of Mr. Z by massaging his ego and autocratically suppressing the troubles in the two manufacturing units under Mr. Y. The result is no bad news comes to Mr. Z and all seems to be going on well under Mr. Y. While all the time lot of trouble is brewing under the surface in these two units but due to autocratic style of Mr. Y all seems to be well managed ( No news is Good news).
Mr. Z , the HOO has the weakness of denying harsh realities when presented to him. For instance, he had hard time believing that his two units have been disapproved by US FDA. His state of denial continued for almost one month and Mgt. did nothing to solve the issues until reality hit one day. Similarly, when the GBP experts would give him some bad news about the units he would counter it by his own ‘good’ understanding of the situation. Denial would put him in a comfortable state where he does not have to confront the harsh realities. The real problem happened, when GBP experts told him that his second in command Mr. Y was responsible for major problems in the two units, he denied it.
Now working with Mr. Z’s psychological issue of denial is the biggest problem to be solved by GBP experts. Unless they coach him to face and confront the harsh realities, all efforts for change will come to naught because power to appoint or remove key personnel is with Mr. Z.
Meanwhile Mr. Y knowing that his game is being exposed by GBP experts will try for the removal of the experts from the company. And Mr. Y enjoys the proximity of the HOO, Mr. Z. He has all the time to keep feeding Mr. Z that ‘experts are not doing good job’. It’s a complex political and behavioural issue which will determine the outcome of behavioural interventions and cultural change depending on the skills and competence of the experts.
In case Mr. Y succeeds, it will lead to exit of GBP experts and another cycle of disapproval-approval of facilities leading to millions of losses. Or else if GBP experts succeed in enabling Mr.Z to face the harsh reality and take corrective action by sidelining Mr. Y, the organization will move on the high path of growth.
Enduring results will come only through tackling the root cause of behavioural problems. The CAPA (Corrective Action, Preventive Action) of behavioural issues have to be implemented in the right spirit.
Can you now imagine how behavioural interventions can save billions of rupees?
Now if Mr. Z takes no decision and the stalemate continues, the GBP experts have a bigger task. Now they have to ensure that Mr. Y’s dysfunctional behaviour in the unit is kept to the minimum to avoid damaging the units. How will they perform this task?
Most important intervention to achieve above is to have excellent rapport and trust of all the employees in the manufacturing units with the GBP experts. This requires unique set of skills. Partly the rapport is achieved through empathic listening and partly through special interventions during training programme of these employees by the GBP experts. Primarily employees build trust when they know that GBP experts look after their interests in a win-win manner with the management as well. Employees, even at the worker level are often intelligent enough to know who is working for the interest of the organization without exploiting them rather in a win-win manner.
Through this rapport and empathic listening emerge the real issues blocking the GMP implementation. From here onwards, the GBP experts constantly provide inputs to GMP experts to handle those blocks and pave the way for smooth implementation of the cGMP on an enduring basis.
The GBP Process Cycle
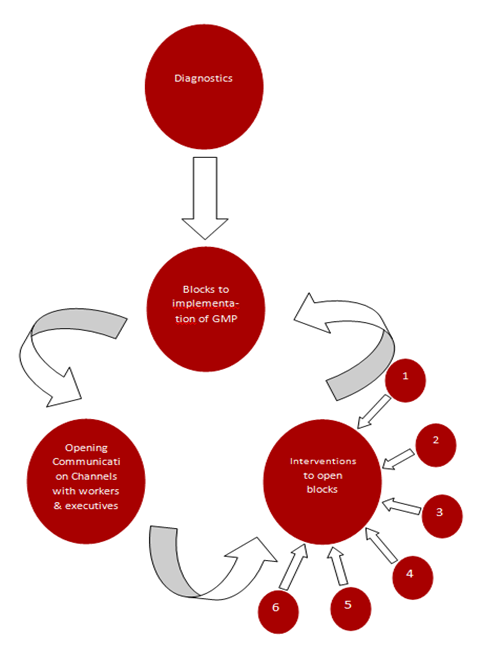
Thus opening communication channels at the unit level with workers and executives is an important achievement for cGMP to succeed. Similarly, executives and workers must get a feeling that top management is getting the reality through this new communication channel with GMP Experts and not what is being fed by the cronies to the HOO to serve their own vested interests.
The above is one iteration of the cycle ( 1. Diagnosing Blocks, 2. Opening Communication Channels, 3. Interventions to open blocks). Minimum six such iterations are required to create learning within internal cGMP experts to learn the art of implementing cGMP on enduring basis.
Many more interventions of GBP experts are required such as replacing old cultural stories with new cultural stories of successful implementation and practice of cGMP. GBP experts write down these stories to circulate amongst the organization wide audiences.
Remember we only touched the resistance to change part only briefly. There are continual interventions to convert resistors into ambassadors of new culture. The resistance group, for example, may even stop the circulation of stories of new cultural change. How to deal with such situations is a continual challenge for GBP experts.
Once the internal system has reached maturity, it is time for the GBP experts to hand over and exit the organization.
Here is what Mr. Gerald W Heddell, director - Inspection, Enforcement and Standards Division of UK's MHRA says "Clearly those (facing regulatory issues) companies have to revisit the way they behave and the attitude throughout the companies"

Good Behavioural Practices
“At the heart of enduring success for any Pharmaceutical company lie Good Behavioural Practices- GBP”
Good Behavioural Practices - GBP emerged from our Behavioural Consulting with Pharmaceutical manufacturing Organizations. There is a business compulsion of implementing cGMP (Current Good Manufacturing Practices) in these companies if they have to get regulatory approvals for manufacturing and exporting medicines.

GBP Audit
It is well established in our experience of consulting with Pharma organizations that cGMP cannot be implemented without first establishing GBP. Establishing GBP requires extensive cultural transformations.
GBP Audit is a proprietary tool designed by UTSAV to enable Pharma organizations understand their readiness and maturity in implementing cGMP on enduring basis.
Read More

Distance Learning Program for GBP
With distance learning programme UTSAV aim to educate the Pharmaceutical executives at senior levels( with minimum ten years industry experience) on behavioural knowledge, skills and attitude necessary to implement GMP and QMS.
At the successful completion of programme, requiring written test and viva voce, the participants will be awarded certificate of completion of First Level GBP programme.
Case Studies
Read case studies of UTSAV clients who used our Good Behavioural Training Programs to drive their growth and build their brands.
A case study is a comprehensive study of a social unit of society, which may be a person, family group, institution, community or event. A case study focuses attention on a single unit thoroughly. The aim is that to find out the influencing factors of a social unit and the relationship between these factors and a social unit.

HR Communication & Newsletters
GBP based communication is a powerful tool to bring about change towards GBP. And it is an art few know how to implement in organization. We have developed a special expertise in the same and many clients have used it to their immense benefit. Please find below the actual samples of communication designed by us for the CEO of a Pharmaceutical orhganization.
Read More

Blog
Good Behavioural Practices can ensure competitive success for Indian Pharmaceutical industry. However, Indian Industrial culture is still to fully come out of ‘Chalta Hai’ mindset. Everyone knows that large numbers of Pharmaceutical plants need vast improvement in the work culture to compete in the world markets.
Read More

Pharma - Behavioural Issues
Pharmaceutical companies face complex issues that grow more challenging by the day
One of the most crucial questions facing the industry, though, is what leadership skills companies will need to navigate this complex and changing landscape—and how current pharmaceutical leaders stack up.
Read More

Video
"When you educate a man, you liberate a man." – Dr. Benjamin Carson.
Dr. Carson, a distinguished man of science and healing, the Director of the Pediatric Neurosurgery Division at the John Hopkins Hospital in Baltimore, talks about political correctness and how it can muzzle an entire nation. What is the point of an education if we do not voice our opinions on important issues?
Read More

 +91 9810009311
+91 9810009311 pradeepprakash2001@gmail.com
pradeepprakash2001@gmail.com





