-
Good Behavioural
Practices (GBP)Our Services
- Organizational Change and Development
- Training and Development
- Psycho Profiling & Assessment
- Performance Management
- Competency Mapping for GMP
- Artificial Intelligence(AI) & Machine Learning(ML) for GMP
- HR Communication Newsletters
- Ethics and Sustainability
- GBP Manual
- GBP Audit
- Distance Learning Program
Technical
AssociatesCase
StudiesBlogs



Case Studies
These are important case studies compiled by our team. Names and data has been changed to hide the identity.
-
1. Autoclaving for Success
-
2. Where There is a Will, There is a Way
-
3. Small Initiatives, Encouraging Results
-
4. Road to Excellence is Paved With Challenges
-
5. FX1 - A Beehive of Activity
-
6. Story of Fall and Rise of Department of QA
-
7. We Will Win
-
8. Glimpses of Our Struggles to Excel in Production
-
9. PSD Biotec: A Constellation of Stars
-
10. Transforming Lives, Transforming Societies
-
11. From Role Taking to Role Making
-
12. Bricks are Made of Our Struggles
-
13. Faster, Higher, Stronger- The Spirit of Olympics
-
14. FX1 - The Mighty River has Started Flowing Again
-
15. Innovation Knows No Boundaries
-
16. Ready to Scale the Last Lap
-
17. Hum Kisi Se Kam Nahin!
-
18. Behavioural Interventions to Unlock the Value Hidden in Pharma Companies: Lessons from Sun Pharma and Ranbaxy Deal
-
19. Values Hierarchy for GBP
Autoclaving for Success
On 7th May, 12 in a presentation to Dr Tarun Chauhan on
timelines for start of production, QA head Sunil Sunariya was
voicing his concerns about starting production on 27th May
because Autoclave validation of FX1 may face issues due to
adoption of International Standards which are quite stringent.
FX1 Autoclave is relatively old and of Indian make while FX4
is new and German make.
"What are scientific rationale for FX1 autoclave not clearing
the requirements? Indian make is no valid reason," asked Dr
Chauhan. Sunil Sunariya started fumbling on this but fears and
apprehensions were high on everybody's mind about FX1
autoclave validation. Now more so after Sunil voiced his
concerns which had silent resonance with others sitting in the
room. Even though some scientific rationale was offered but it
was more of rationalization than real science. Meeting moved
to other issues but the anxiety of clearing FX1 autoclave
validation kept hanging heavy in the minds of members present
there.
And finally, when the autoclave validation process started on
10th May, the expected fears started taking reality. The
cycles of validation could not qualify one criteria or the
other and failed. Engineering started running its engineering
cycles but it could not bring the parameters in required
range. On Monday, 14th May, Ankitesh from Gurgaon unit and OEM
Engineer from Hyderabad also came to sort out the technical
issues but the problem continued for next two days. No headway
was visible. The OEM person was recommending new PLC and other
tubings but the same would take time. Such solutions cannot
solve our problem at this moment.
Once again at morning meeting gym, the air was heavy with
apprehensions. There were serious voices asking for change of
acceptance criteria in the protocol so that FX1 autoclave
would pass validation. Engineering person declared that
because autoclave is old and Indian make it cannot pass the
European acceptance criteria laid down in protocol. At that
moment one member asked,†Have you ensured that all your
engineering inputs and machines sensors etc are working as per
requirements?" This spurred Robin to go in details of the
input conditions of steam, pressure etc. The decision taken
collectively after all the discussion was that we should
examine in detail all the engineering inputs and parts and
conditions before blaming the Autoclave and its Indian-ness.
The engineering runs started in all earnestness again. Large
no of cycle runs were taken by varying different variables to
rule out all kinds of technical faults.
Next day Alexander is here. When engineering says that they
cannot bring the acceptance of equilibrium time within 30secs,
he simply refused to dilute the acceptance criteria and stated
categorically, "I have done validation many times myself and
in 1993 brought the equilibrium time to 15 seconds in an
autocalve as old as of 1980. Ask me for help if you need any".
And then he guides him to check the vacuum and so on.
Engineering gets to work again; struggles with more cycles,
installs the autopressure control valve, manages the jacket
pressure, installs sensors of different make and so on. There
is a bright message in our email inboxes on Sunday, 20th May
at 9:07 am. The equilibrium time is now below 30secs! We are
successful in meeting the international standards for Indian
make autoclave, few years old.
However this is one hurdle before other have to be crossed
when we can say we are fully through with our autoclave
validation as per best specifications. The next test is to
pass the load with garments followed by the Liquid load.
You might have guessed by now that our engineering team along
with production executives will achieve that too. And let me
inform you that a week ago despite many engineering issues,
Garment load has passed. We are waiting for the news of liquid
load passing today as I am writing to you.
All have confidence that our teams in FX1 and engineering will
make it happen. They are oozing confidence and have set
standards for other units to achieve these benchmarks in
autoclave validation.
Same Indian old autoclave is meeting world standards when our
teams have decided to overcome all challenges! We can do it!
This is the story of our new culture of excellence. Alexander
not accepting to dilute the specifications of acceptance and
insisting on meeting the international specifications
criteria, engineering meeting that challenge and FX1 team
working day and night with engineers did all of us proud! Our
confidence in our abilities to be world class in our standards
has gone up. We are proud of ourselves and well on our way to
becoming world class!
Where There is a Will, There is a Way
The new culture of Zero Tolerance for non-compliance with QMS
guidelines is bringing many changes in the way work is being
done at Bangalore these days. One of the manifestations of
this change sweeping the work culture was that production
Heads of facilities FX1 and FX4 were clear that autoclave
validation start will not happen till steam has been validated
to be within the specific parameters defined by International
standards. Till now nobody could tell when the steam was last
validated in these facilities and therefore it seemed fraught
with unknown difficulties. What if pure steam fails this
validation? What will happen to the timelines for start of
production? Many such apprehensions were hanging in the
environment. But we must start the process and see what
happens.
External experts to test the steam were called and when the
same was tested in March,12, it was found that pure steam had
many parameters out of range including non-condensate gases in
steam above the specified limit of 3.5% or so. The struggle to
deal with the problems started in right earnest. For instance,
debates on installing and ordering degasser and various heated
discussions on time it will take, costs etc kept the struggle
on without reaching any conclusions. Dr. Shashank Jain even
suggested that since non-condensate gases beyond 3.5% is not
going to affect the autoclave validation, we can go ahead with
it. But the team at the site decided to go ahead with solving
steam issue rather than compromising on steam validation.
Engineering had to once again go about finding the root cause
of the issue. No dilution of standards now, the production
team at Bangalore was clear!
Engineering had the hints of problem because the garments
sterilized by steam used to be wet ( and this problem had
existed for long without anyone trying to solve the same is
another story). That means condensate was mixed with steam.
When they went about finding further why it was happening, a
great discovery happened. There were no steam traps anywhere
except where the steam cycle terminated! And that is why the
garments sterilized were getting wet.
The hurdles to solving problems did not abate. For example,
Pradeep Periwal suggested for three way valve. HP Saini,
Engineer-in-charge of site shared the limitations of the
solution: it will take two months to get the valve and even
then there is no guarantee that it will work. Other solutions
had to be found. And when pushed further for finding the
solutions, engineering team tried few more solutions. They put
steam traps at some of the points of the steam path. Eureka!
It worked! The gases also escaped along with condensate at
these traps.
When they checked the pure steam quality by the testing kit in
house (bought by them by now instead of depending on outside
help), it was a moment to rejoice. Pure Steam passed all the
tests including the non condensate gases limit of 3.5%.
The lessons are clear: Our teams can solve problems when they
focus their energies on it. Our new culture of Zero Tolerance
for Non-compliance with QMS requirements is giving rich
dividends in the form of high confidence on our own abilities
to achieve full compliance with requirements. Last but not the
least, now we know, nobody can stop Team Bangalore from
getting PQ from WHO in August.
Small Initiatives, Encouraging Results
When Dr Piyush joined Animal house in January this year (2012)
as Head, he had few cultural shocks. Things were moving at
snail's pace; getting resources and basic stuff like laptop
took good amount of time and effort. However, things started
changing and soon he figured out ways to get things done fast,
including in his own department. He found some inefficient and
unproductive practices of Animal House unacceptable. For
example, the farm meant to produce grass for animals was
producing nothing despite one man fully deployed to do that
work.
Dr Piyush made further investigation and discovered that the
man deputed to cultivate grass was not very efficient person.
Soon he made enquiries from his own staff about skills of
cultivation in any one of them. Yes, there was one person who
was good in farming and could give results.
Quick action produced good results. The new man, Rupam started
giving results with all the support of fertilizers, water
pipes, tractor etc from Admin team headed by Mr Vipul.
Moreover, he only spent half a day and not full day. From nil
production of grass in February, they produced 30 kgs in
March, and 70 kgs in April. In June, it has touched 120kgs.
Animal House is confident of meeting their requirements of 300
kgs from the captive farm very soon.
It has saved them the expenditure incurred on buying
vegetables for animal food. When full requirement will be
produced in captive farm, the saving will touch few lacs per
annum.
The above is just one example. There are more examples where
small initiatives have resulted in big savings for Animal
House. The new vendors identified for supplying Guinea Pigs
will result in savings to the tune of Rs thirty two lacs per
annum. Similarly for Corn Cob purchase, the saving will be
about Rs six lacs per annum.
Lesson is loud and clear: small initiatives can produce big
results provided we take ownership for our department,
resources and results.
Good work Animal house team! Keep it up and inspire others to
be as innovative in saving costs. Small drops will add up to
make PLS ltd tide over difficult times soon.
Road to Excellence is Paved With Challenges
Our audited financial results for Financial year 2011-12 have
just been published last week. When I look at them, it is
clear we have suffered a big financial blow due to loss of
Vaccine business after WHO PQ fiasco. We have moved from Rs
120.67 crore net profit during 2010-11 to a net loss of Rs
180.65 crore during financial year 2011-12.
And if I look back in the last decade or so we have been
having a continuous growth, almost doubling our revenue growth
in last five years. Naturally, we cannot take this hit lying
low and need to examine our own selves and to discover what
all we did that led to this. I do not intend to blame either
others or ourselves for this but use this as an opportunity to
find our weak spots and deal with them so that it never
happens again to us in future. Our vision of 2020 is firm and
this hit we take as a wakeup call to strengthen our muscles to
achieve what we have set our goal in 2020 vision.
Success, if comes easily, loses its charm. It neither develops
character of men nor it increases the hunger for further
growth nor does it develop confidence. Therefore I welcome
this bad patch in our growth as a nature's exercise for us to
develop our muscles to achieve greater success.
Look at our few success stories at various sites. Let me site
few from our epicenter of all activities for WHO PQ,
Bangalore. We never thought we could meet international
standards in Autoclave validation specifications for our
Indian make, relatively old autoclave. Our engineers and
scientists at Bangalore have done precisely that. Look at
their confidence after this success against all odds. They
have this confidence because they overcame odds; it took them
good number of days and nights of innovative trouble shooting
to achieve that.
Another story of steam validation at Bangalore proves this
point again. It seemed like an impossible task when they began
doing pure steam validation. But soon with their untiring
efforts they could meet the specifications during pure steam
test.
Many such success stories being written at Bangalore are
proving beyond doubt that this hit is a blessing in disguise.
We never knew our potential was so high. I am every day
discovering our bright talent which I never knew existed with
us.
All the same, I have also come across some cases of QMS non
compliance which we have dealt with firm hand. Our policy of
Zero Tolerance for unethical 'behaviour and reporting of data'
has sent a clear message across to all that we mean business
and past mistakes will not be repeated.
We have a firm mandate from WHO in their March,12 letter that
we have to have a culture where excellence is the norm and
unethical reporting is a taboo. We are implementing this in
all seriousness and this is what I want to see at all sites.
Excellence in all forms will be encouraged and rewarded. At
the same time, mediocrity and unethical behaviour will have no
tolerance.
In the end, I am reminded of famous words of Winston
Churchill, ex PM of England who took UK to victory during
World War under extremely difficult times: "I have nothing to
offer but blood, toil, tears and sweat." To win this battle of
WHO PQ under similar circumstances, I would like to say the
same to you today. You may have to give your toil, tears and
sweat and in the near future you may not expect rewards due to
financials mentioned above.
I am fully aware that to achieve this culture of excellence
and making Bioline Ltd a highly respected Pharma company
globally is a very challenging goal for us. But through this
agni pariksha of WHO PQ audit and our resolve to excel, we are
going to emerge stronger and more successful to continue our
journey of excellence.
FX1 - A Beehive of Activity
In February when we met Gaurav Gandhi, Praveen Arora , Madhur
Sehgal and their team they were going through a phase of high
uncertainty. The dominant feelings were were of anxiety and
stress. They felt like being in a long winding tunnel with
light at the end not visible. Many things were open but
closure was not in sight; rather more issues were being opened
than closed every day. At times various tasks had to be
reworked because some issues required inclusion or revision.
Today at the beginning of June, things are having a different
flavor. FX1 is a beehive of activity with busy-bees flying in
and out of various rooms. The energy levels of the department
are high as can be seen by flurry of activity going on there.
Autoclave development cycles have been completed successfully
and validation process has started yesterday (you might be
aware through our earlier stories, FX1 has been able to meet
specifications criteria of European standards for its Indian
make autoclave during cycle development and set benchmarks for
other sites), all the batch records of USP and support area
have been made more user friendly to reduce the unnecessary
rigidity in the same. The same records are being revised for
DSP along with related support area records. It will make the
functioning hassle free and efficient while at the same time
strictly QMS based. It has been a huge task revising the Batch
Records with excellent inputs from Martin and other
consultants.
Deviations are being closed (with quality rigor) almost on a
daily basis and progress monitored so that before production
starts in mid June, all the essential CAPAs are implemented
and deviations closed. There are certain competency
enhancement requirements, as pointed out by Robert, in terms
of filing and recording deviations and CAPAs. Also increasing
rigor in terms of executing CAPAs on the ground is a continual
exercise that FX1 executives will have to sustain.
Various executives from other departments have shared that
attitudes of executives in FX1 is cooperative and excellence
oriented. They are clearly much more QMS compliant than a few
months ago. Once or twice evidence of non compliance occurred
in the past month. When the same was brought to the notice of
FX1 head, he immediately took action to rectify the same and
ensured that such non compliances will not happen again. The
confidence of even FX1 workers was clearly high. It was
evident during our interactions with them in last few days.
All the FX1 team members are now waiting anxiously to get the
facility audited and cross the hurdle of WHO PQ. They have
been working long and hard since last May, 2011 (before the
earlier WHO audit). The postponement of NRA audit due on 28th
May was also a disappointment. "When we are ready for it,
waiting seems so long," commented Shobit Shukla when I met him
on 28th May. Now they are waiting for June 18th when NRA will
come to audit the facility at Bangalore. They are seeing it
like a practice match before the finals. However, at the
moment the focus of FX1 team is on starting production on 15th
June, closing the CAPAs and deviations and completing revision
of BMR at the earliest. The feelings are the same when a well
prepared team that had lost the last world cup is straining at
the leash to prove its mettle and restore its honor.
Story of Fall and Rise of Department of QA
"Quality Assurance department is doing everything except what
it is supposed to do," was a shocking comment from a senior QA
professional who joined QA department in Bangalore in
December,2011. QA was doing calibration of equipments /
instruments at site (more than 2000 in number), doing
validations besides doing its hard core work of QMS
management, vendor qualification, batch release,
documentation, IPQA and so on. Extreme case evidence was an
SOP of QA for 'maintenance of the cleanliness of toilets'
which should actually belong to Admin.
All this was taking its toll on QA executives. Their
performance was getting affected and so was the quality of
work. For instance, I heard Rajesh Gupta, then head of FX1
comment to me in February(2012), "I have sent third reminder
to QA for calibration of XYZ equipment and if it is not done
by tomorrow, which is the last date, we will not be able to
use that equipment and our work will come to a standstill."
Things had to be sorted out otherwise QA's low effectiveness
will affect the entire site. It led to Dr Rohit sharma, then
VP Corporate QA organizing an expectations sharing meeting of
QA head and his team with other heads to bring more clarity to
QA's roles and responsibilities during third week of March. It
was a great step that led to QA department shedding all roles
and responsibilities which did not belong there. That included
calibration to be done by Engineering and validations to be
done by respective departments. It was decided to transfer all
such tasks to their respective departments by 31st March,2012.
Some issues like Protocol responsibilities lingered on for a
while but eventually shifted to respective departments by
Francis's intervention. QA in its new avatar was leaner and
sharper in its focus of work. It had a clear mandate of
ensuring implementation of QMS in letter and spirit. No
violations of strict QMS processes will be tolerated. Of
course people needed to improve their competencies to file
incidents, deviations, change controls, and then implement
effective CAPAs.
Monthly Quality Review (MQR) meeting of May(2012), reflected
the definite improvements in the working of QA department
during the previous month of April. The number of incidents
raised in April increased to 1089. Similar increase happened
in deviations (29) opened and CAPAs (16) raised. IPQA became
very active and ensured that no non compliance went
unreported. CAPAs implementation on ground was verified by QA
and only then their closure happened.
For quite some time now, QA is daily flooded with documents be
it SOPs for review, Change Controls, CAPAs, deviations,
incidents, implementation reports etc from all the
departments. QA executives are submerged in documents and
actions to close the issues related to documents. It has
become a centre stage of action happening at Hyderabad site.
Executives in QA, sometimes succeed and sometimes fail in
satisfying its internal customers. One does hear occasional
complaints of issues getting dragged, back and forth movement
of documents, differences of opinion between executives of
other departments and QA.
The empowerment of QA department along with its
responsibilities of ensuring strict implementation of QMS of
cGMP has been a long and arduous journey (and it is still on)
where executives of all departments including QA are slowly
but surely getting used to. Those who understand QA say that
executives and workers in other department should respect as
well as fear QA for violating any QMS provision. This cultural
change is the one desired by WHO in its letter of March,2012
to CSR. The quality system has to be the robust one which will
ensure patient safety.
On 12th June, during MQR meeting the signs of change were
visible again. There were 611 incidents (raised and
satisfactorily dealt with), 24 CAPAs and 72 deviations in the
month of May,12. The production related open CAPAs will be
closed before production starts in mid June.
It is good to have deviations but care has to be taken that it
is not due to resource constraints but due to process and
product issues. For example, HVAC revalidation should not drag
beyond June end otherwise deviation for use of HVAC after June
will be taken and this is what should be avoided. Executives
in other department should get serious in getting HVAC
re-validation completed before June end and sort out all
issues of PR raising, budget etc in this regard. This is the
meaning of 'people should respect and fear QA' and comply with
all QMS requirements. I believe this empowerment has happened
in QA and will be fully internalized by all before WHO audit
in August.
Hard work of QA team is finally visible. However, one cannot
rest here; the journey is long and arduous. The competencies
of people to understand and implement processes, file
deviations/change controls, CAPAs (and implement them)etc has
to be continuously raised. The improvements in system and
people should be visible to everyone including the WHO
auditors who are expected in August.
Dear QA, you are important to us. Our future depends on how
well you carry out your responsibilities!
We will win
During March, 2012 after the Gurgaon facility audit failure the morale of ADB teams was at its lowest nadir. Nine months after the Bangalore facility delisting, Gurgaon debacle was the biggest business and professional shock for all in ADB. The letter received from WHO in March, 2012 had many unkind statements about the state of facilities in ADB along with work culture here.
Coping and recovering from this setback was not easy. The dark clouds of hopelessness were hanging heavy in the air. Convincing WHO authorities to have faith in our abilities to bounce back and come up to their expectations in terms of our compliance with QMS of cGMP as well as work culture of excellence seemed almost impossible task.
To rise to this challenge required rare courage and never say die spirit. With good PR, a small window of opportunity was opened when WHO authorities agreed to meet the top management in Geneva on 16th April, 2012. A team consisting of top management including Dr Abhijit Bose, Mr Santosh Kumar Maurya, Dr Vivek Pathak, Dr Shailesh Kumar Pandey, top QA personnel from Bangalore and Hyderabad along with other experts with their collective wisdom finally met the authorities. It was a moment charged with high tension. If this opportunity to convince WHO to come and re-audit goes, then ADB will have to wait for next three years to get back to business in Easyfive.
Your guess is right. The top team came out successful and WHO agreed to re-audit in September with some gates of experts’ audit and NRA audit.
And now in June comes the first Gate audit of Dr Riaz Hussain (from 15th to 18th June, 2012) followed by second gate audit of NRA (18 to 20th June, 2012).
There were mixed feelings after Dr Riaz Hussain audit on 15th June. The good news was that they will ask WHO to come and audit the facilities of ADB in October first week. The reason for that is ADB teams have made sincere efforts to excel in GMP compliance and are making good progress compared to where they were in July, 2011. The not so good news was it was a conditional clearance. Good amount of work is yet to be done as per CAPA submitted to WHO in April, 2012. Therefore ADB facilities will be re-audited by international experts once in July and again in August before final invitation to WHO.
The feelings are one of relief and joy after the NRA audit. They have expressed satisfaction with some observations. This paves the way for WHO audit to be held in October, 2012.
The ADB teams at Bangalore and Hyderabad deserve pat on their backs for reaching thus far after the July debacle. The task that once seemed almost impossible to reach is now almost within reach. Just three more months of hard and sincere work to restore the honor lost in July, 2012.
You will win ‘Team Bangalore’ and ‘Team Bangalore’! Who can doubt that now?
Glimpses of Our Struggles to Excel in Production
The good news from Bangalore is that production has commenced
in block FX1 during last week. Block FX4 too has commenced
production this week. Behind this news lies the months of
struggles to bring these units excellent in their facilities
as well as documents.
Overhauling the Batch Master Record for these facilities has
been a mammoth task under Ankit's leadership. Everybody is
proud of the new BMR as it captures the production processes
and issues resolution in these documents so well. "It is idiot
proof," Tyagi tells me with a childlike pride in his tone.
Even Mr Saibal Mukherjee from Hyderabad told me that these BMR
documents are really a quantum leap for PSD Biotect production
facilities. Behind these BMR lies the hard work of Namit,
Anuj, Shubham, Sudhanshu, Rajat, Swagat and their team.
The autoclaves of both FX1 and FX4 have been validated,
meeting international standards. Priyanka & Abhra in FX1
and Sonali & Bhavin in FX4 have worked day and night for
weeks along with engineering to make this possible. There were
enough anxious and despairing moments due to sensors or Kayee
Validator malfunctioning or steam pressure variations etc
before they were finally validated. How can we forget the
struggle to get the pure steam with condensate gases within
limit of 3.5%? It took few weeks of experimentation and
innovation before it was finally achieved.
Cleaning validation in FX4 was done more efficiently using few
innovations learnt from Gurgaon facility. It helped save few
days. The real anxious moments happened when the differential
pressures were not being maintained in FX4 facility. It
required quite a few iterations of innovative thinking,
planning and execution of team consisting of engineering,
production and Technical Consultants to finally achieve that.
The above is just a glimpse of the ground level struggles of
executives in production and engineering departments to make
these facilities world class. These struggles have been etched
indelibly in the history of PBL towards its march of
excellence and Vision 2020. PBL salutes these heroes, sung or
unsung, for bringing these facilities to this level!
PSD Biotec: A Constellation of Stars
At Hyderabad and Bangalore, the scorching and dry heat of June
has gone and heavy downpour of July has begun. The wet smell
of earth permeating the air is a comforting change after the
'parched and dried' feel of the summer. The richness of green
in the freshly washed trees is refreshing to the eyes.
Similar is the feel when we go to Hyderabad Warehouse now.
After Mr Piyush took over as head of the Warehouse in June,
the smell and feel of the same has changed. The surroundings
are clean, the documents are as they should be, and people
energized.
He took many initiatives including inventory cost reduction,
starting two shifts to serve customers from 6am to 10pm,
organizing solvents store getting pallets from VFP, Gurgaon
taking help of Mr. Abhijit and Mr. Riaz there. Dr Alok was
equally impressed when he visited Warehouse two days ago, "I
found his approach to work very diligent and sincere". The new
head in warehouse is a welcome change.
Somewhat similar refreshing change is visible in Hyderabad QA
after Dr Santosh took over as head of QA department in May
this year. Since May, 290 SOPs have been approved, more than
200 CAPAs and 145 Change Controls have been closed and close
to 170 documents of new Batch records, Production orders and
Cleaning records have been finalized and approved. "There is a
major shift in the way QA is facilitating production since
May," said Praveen Kumar, Head Production FX1.
PSD Biotec is a constellation of Stars, who keep shining
brightly, whether they are noticed or not.
Recently Dr. Prabhat Kiran's team members won a prestigious
award for publishing a paper:
"Congratulations to Conchi and her co-authors on winning the
Shepard Award in the assessment category. The Shepard Award is
to recognize excellence in scientific achievement by CDC and
ATSDR authors of outstanding scientific papers," emailed
Rebecca, announcing the award.
Let me share one more example of a Star in PSD. One fine
morning Dr Raja Kumar received an email from leading
Nephrologist, Dr Pallavi praising a PSD Star's outstanding
service. "In particular I would like to mention Mr. Bhuwan
Bhaskar who diligently worked at the conference and made our
attendance very comfortable," wrote Dr Pallavi. Stars are
shining eternally whether noticed or not. But they make the
sky what it is! What will be sky without them?
So do our stars in PSD. They make all of us proud of what PSD
is today!
Transforming Lives, Transforming Societies
When Robert Koster visited Hyderabad Warehouse for audit in
June, 2012, he discovered cobwebs on the roof adjoining
warehouse room. And he highlighted the same to our JMD, of
course to his utter embarrassment. How is it that an auditor
notices such cases of carelessness and not our own persons?
This led to the revival of religiously making point
observations everyday by HODs at both Hyderabad and Bangalore.
Let’s eliminate non GMP observations ourselves, rather than
being pointed out by others and especially so by auditors.
This seemingly simple initiative hides within it the
profoundest of attitude change which makes people live a happy
and productive life. Can you guess it?
Taking ownership and responsibility for my area, department,
company, family, society, nation and the world!
It seems more comfortable to be careless and take no
responsibility, but the price of that comfort is suffering for
life. Discomfort in the short term, but joy in the long run is
the choice of people who take responsibility and ownership.
This is the fundamental and profoundest secret of successful
living.
And results of this drive on Point Observations have resulted
in many benefits in the short span of three weeks. New
Fermentor worth three crores lying unutilized in Hyderabad
will now be either utilized or converted into cash. This
observation and action to bring Fermentor back to MM fold was
done by Mr SP Saini.
And a serious point observation came when Rahul Sr Manger QA,
in Hyderabad went to QC and discovered that one of the
scientist was making two page report of EMP plates done on 6th
July (reports being made on 12th July) from his memory without
consulting the raw data. A serious ethical lapse caught well
in time. This became a critical incident to send message
across to all about Zero Tolerance for unethical reporting.
Many such issues are being rectified well in time due to this
initiative, day in and day out.
Being vigilant, by taking responsibility for our own affairs
will not only benefit organization but our personal lives as
well. When I go home now, I make point observations at home
too and take suitable actions to rectify the situation, be it
removal of dust from tube lights or repairs of broken window.
Our personality is being shaped by our organizational culture.
Our transformation will lead to transformation of our families
and societies. When we are being more scientific &
responsible, self disciplined & hygienic in our habits, it
is but natural that our families will acquire such attitudes
and prosper.
And this transformation is taking shape in MPS Ltd, slowly and
quietly.
Heartfelt Thanks to all who are championing this
transformation in MPS!
From Role Taking to Role Making
The rainy season has come but rains are playing truant. The expectations of good rains are slowly transforming into a frustration of long wait for clouds to shower heavy rains. The clouds often come but they leave behind people longing for rain.
Similar longing was there in many people to see the facility at Hyderabad, absolutely neat and clean, but it seemed a farfetched goal. Many times it was resolved to make it sparkling clean but it remained a pious hope. Even Mr Rohit Dave took the lead in taking around with him Ritu Basumatari, Alok Surya, Deepika Panday and Gajender Singh and started Safai Abhiyan as well.
Last week when Mr. Alok Surya and Mr Ankit were going past utility area, they were upset to see the area littered with leftovers like iron rods, drums and such miscellaneous items thrown carelessly here and there. Something had to be done, thought Mr Surya. Moreover, most of the waste thrown belonged to engineering surplus left by people after working.
Next day Mr Surya organized his entire team of more than 50 persons on duty including the new AGM and sent them for two hours from 9:15 till 11:15am to clean up the entire area near Utilities. It took them couple of days of work to not only clean that particular area but also the roofs of different blocks and other difficult to reach areas. It has become a regular practice for engineering team to go five days in a weak and clean up the areas as required.
This not only makes the facility clean, it also instills in them the discipline to clean up after their every activity in the plant and keep the plant absolutely neat and clean. When next time after two days, Mr Ankit walked past that area near Utilities and found it spick and span, he was overjoyed. It seemed like miracle had happened in two days. He was so impressed and happy, he immediately gave a note of 500 rupees to his aide and got laddus to be distributed to PDS employees in celebration.
And it rained heavily too this week around Hyderabad; ending the wait for showers!
It is a perfect example of how one can enlarge one's role to do whatever will add value to the organization. This shift from 'role taking to role making' is visible at various other departments and units as well now a days.
Take another good example from Bangalore. The assets of residential flats in Bangalore were not being fully utilized because of certain policy issues. Some proactive action to initiate changes in the policies and it resulted in saving huge amount for the company when these flats were utilized to accommodate PDS Bangalore employees. When people start becoming proactive and enlarge their roles by going beyond their normal scope of work, they not only add value for company but also get huge job satisfaction and earn goodwill of management.
And it is just a matter of attitude!
Bricks are Made of Our Struggles
Every day I see NDL Biotec workers, staff, executives,
managers and leaders grappling with issues related to managing
production at Bangalore as well as Hyderabad. And the
situation must be similar at other sites, units and even
corporate office. Are they just fighting to resolve problems
which they are encountering or it is much more than that?
Often the struggles have to do with engineering maintenance
problems, hiring, retaining and developing effective people,
procuring materials against challenging circumstances,
managing cash flows, changing mindsets as per QMS culture,
attracting and getting customers, stabilizing and excelling in
work related processes, resolving people conflicts, keeping
high morale and so on. The list is endless.
There is pain in all these struggles. This pain I see when MM
is asked to procure material in urgency from vendors whose
payments have been delayed and are demanding payments. This
pain I see when production executives in FX1 and FX4 at
Hyderabad and Line 1 at Bangalore work 12 hours a day normally
and exceptionally even stay continuously for 70 hours ( they
bring their clothings along for three days). This pain I see
when engineering executives at Bangalore and Hyderabad have to
cope with bringing the facilities under compliance in
temperature, relative humidity and differential pressure
despite many technical challenges. And when, QA has to ensure
that people adopt QMS culture and comply on their own with all
requirements without follow up. And when, QC has to cope with
large number of tests required in validation and routine
activities with limited resources. What is the meaning of all
these struggles? The imagery that comes to mind is one of
building a temple brick by brick. While the brick is being
placed we may not feel the enormity of success that is
awaiting us in the form a grand temple, but it surely is going
to create that.
And our struggles are bricks that are creating a world class
biotechnology organization which will prevent or control or
cure millions of people from deadly diseases. Isn't that
reason enough to feel proud of our everyday struggles.
Faster, Higher, Stronger- The Spirit of Olympics
The world is witnessing human spirit's aspiration to break old
limits and set new records of physical and mental prowess at
London Olympics. It is in the 'Collective Unconscious' of Man
to aspire for and achieve new records in all human endeavours.
Sporting events provide us a glimpse of man's life in a small
capsule of few hours to few days. One has to play as per rules
to win using one's skills, knowledge and attitude. Be it
cricket, football, hockey or athletic events. In fact team
events like Football or Hockey matches provide perfect
examples of how we work in teams at our sites. The Captain has
to take the strategic decisions and ensure that each player
knows his role and is able to synchronize with the rest of the
team. There is sweat and tears inherent in struggle to win the
medals by perfecting one's skills, day in and day out, year
after year and by every player of the team. And then the day
of match decides the fate of the teams! If you win, great;
celebrate the win. And if you fail, shed tears, gather your
courage again and go back to practice field to perfect the
skills, this time with much more vigor, as the honor has to be
restored.
Medal winners will necessarily have many failures behind.
Failures provide the energy to bounce back and perfect one's
skills and give motivation to do or die. Look at what Usain
Bolt, the Gold medal winner for 100 meter sprint in London
Olympics, has to say, "When Blake defeated me in trials during
July,12, it was a wakeup call for me." Does the above imagery
rings a bell in our hearts somewhere?
The wakeup call for CSR Biotec has come through audit of
Robert in July. The efforts have to be re-doubled by one and
all in the teams. The QMS has to be perfect or near perfect
when the WHO audit team comes here in October. 'QMS before
Tasks' is the new slogan.
Let the Olympic Spirit of Faster, Higher and Stronger be CSR
team's inspiration!
FX1 - The Mighty River has Started Flowing Againy
We had been waiting for this day for many months. On 14th
June, FX - I at Bangalore re-started production after a gap of
almost one year, post WHO Audit on June 11th. It was a great
feeling across the plant that we are back on production.
However, every day brings along new challenges; from managing
shift wise manpower to keeping the machines running despite
technical glitches. For instance, one fine morning the CIP
machine stopped functioning; its heater conked off. And the
heater is not available because manufacturer has discontinued
the model. That was when engineering had to find innovative
solution to get it fabricated against all odds. And how to
manage the delay because holding the batch midway is not an
easy challenge? One can imagine the anxieties and pressures
that FX1 team has to undergo while giving a successful batch!
The team, consisting of Abhijith, Amen, Krishna, Moxa,
Shalini, Nimisha, Neha, Vipul, Raja, Shilpi and led by Alok,
Bhavin and Robin, has been working relentlessly for 12 hours
and more, day after day, to ensure that we are meeting all the
GMP requirements and follow QMS religiously while producing
successful batches.
While managing each and every challenge well within QMS every
day the D' day arrived. On 29th July 1st batch achieved its
final destination; the batch completed its tortuous journey
and will now be tested in QC for its efficacy and other
parameters. There was anxiety, apprehension and prayer in
hearts of production team as well as others at CLS Ltd, while
the tests of the batch were in progress in QC. The success of
first batch would be the culmination of year of relentless
hard work.
Finally the great news arrived. The batch is meeting all the
criteria successfully. Relief and happiness was all around.
That was first batch; first delivery is always the most
important one; it gives high anxiety as well as excitement.
By now, three batches have been manufactured successfully.
Fourth and Fifth batches will soon complete their journey by
next week or so.
Congratulations FX-I Team. Long awaited success achieved with
great support from all functions. You achieved the same with
full compliance to GMP and QMS, and that's more commendable!
When huge efforts and struggles lie behind our endeavours, can
success be far behind?
Innovation Knows No Boundaries
Entrepreneurship is innovation - applied to satisfy needs of
the customers, wherever they may be. This week we saw an
excellent example set by the top team of our management when
they signed an agreement with Ostimic, Netherland for jointly
developing and marketing twelve products from our labs
Bangalore, Hyderabad and Hyderabad, Mumbai. However, we have
heard only the tip of the iceberg. Won’t it be good to know
the huge ground work that went behind this successful and
win-win alliance with Ostimic?
It all started when the Business Development team led by Mr.
Sudhanshu Shekhar and his able team members Raja Kumar and
others along with Legal and Finance teams of M/S Aman Modi and
Shailesh Panday started working and negotiating with Sudhanshu
Shekhar team led by their Business Development counterpart Mr.
John and their CEO, Mr. Francis. It was on an innovative idea
of partnering with PBL even at the stage of Research and
Development of products right until their marketing in US and
other territories where Ostimic has presence. Rarely do
Organizations take the risk of partnering at R&D stages of
pharmaceutical products. And this is where the top teams from
PSD, ADC and Corporate of CSR biotec got their act together.
It was a great team work led by Dr Pawan Sharma when Ostimic
team came to visit our R&D Centers to assess the
feasibility of products under development seeing the light of
the day. Ostimic was so impressed by the work being done at
Bangalore R&D ADS that they cut short their visit from
three to two days. All the documents were of impressive
quality. Kudos to Dr. Vipul Gupta and his team for tireless
efforts in developing products by strictly following the QMS.
Ostimic selected four products from their site for joint
development and marketing.
Similarly in Hyderabad PSD, Ostimic selected fourteen products
under various stages of development. This is a grand success
of Hyderabad R&D Center, PSD, true to its name!! We share
our deep appreciation of Dr. Vinit jain and his team for the
same.
Vibha Joshi, who led the IPR activities along with her team
members Shivani and Geeta, says it all when she said, "In my
career of 15 years in Industry and majorly after being
associated with other Pharma Generic Industry and knowing this
Business and its ways of working, I can say with conviction
that the deal signed with Ostimic is one of the best, in all
aspects, from technical, business & financial aspects.
Such a kind of deal for Generic products is unprecedented".
There were tense moments when the date of signing got
postponed and due diligence was further extended by almost a
month. There were many a slip between the cup and the lip.
Anything could go wrong and anything could have been the
outcome. We could sense the disappointment in Dr. Pawan
Sharma’s eyes when the team Ostimic left without signing the
agreement due to some technical issues one month ago. Just a
day before that Dr.Sharma had so excitedly shared with his
team that the agreement will be signed next day. But the CSR
Team did not allow this to dampen their spirit and all of them
continued with their efforts and responded satisfyingly to all
further queries from Ostimic. During the due diligence the
Ostimic team had more than 4-5 rounds of interaction with IP
Team and R&D Team. Telecons, Videocons, e-room creation,
data uploading and personal visit for Due Diligence, all
formed part of their evaluation of feasibility. On IP aspects
the team provided Ostimic with detailed IP strategies, 100's
of in-house prepared reports, summaries, presentations and
conclusive opinions, which convinced them of the meticulous
and detailed work done for all these projects. On R&D
front the scientists convincingly provided detailed MS Project
timelines and planning.
Finally, after all this relentless efforts and patience and
perseverance, the Joint Business Alliance Agreement got signed
on 22nd August, 2012.
See what Mr Francis, Head of Ostimic Team has said about CSR
Biotec in his email yesterday:
"I too would like to congratulate everyone who has worked so
hard and diligently to make this collaboration work. We at
Ostimic believe that Panacea will make an excellent future
partner. Our experience with the CSR management has already
been excellent."
It is a proud moment and we all have added another feather in
our cap!
Last but not the least, we salute Satyaki and his team for
delivering great business value through this innovative
win-win deal.
Does Innovation Know Any Boundaries?
Ready to Scale the Last Lap
Now is the time to move from corrective action (CA) to
preventive action(PA). We have produced five successful
batches of HEP in FX1 and first successful batch of PRP in FX4
is awaiting just one more result from QC. Media fill has
started in Bangalore for Line 2. Things are moving in the
right direction, but not without hiccups and sweat of course.
Proactive actions to prevent mistakes are the need of the
hour. Take few excellent examples of creating idiot proof
processes from Animal House.
a)To prevent mistake in dilution of disinfectants and wastage
of the solutions by workers, they are being supplied
premeasured quantity packs for mixing in containers containing
exact quantity of liquid.
b) Broom sticks and Mop sticks have been labeled Species wise
so that the same will not be used in other rooms with
different species.
c) Inclusion of many Pictorial/diagrammatic representations in
SOPs to improve understanding of the reader along with their
Hindi translations.
Similarly Animal House has introduced a New Breeding Policy of
Guinea pig in April 2012 because of which the yield has
increased from the month of July 2012 and is expected to get a
yield of 4000 Guinea pigs by March 2013.
Other departments have been also proactive likewise. The new
priorities in pro-action also include keeping our leaders and
their teams in excellent mental and physical shape. Pre WHO
audits by PCS experts is another opportunity for us to act
proactively to resolve any possible issues.
Teams (Vaccine) Hyderabad and Bangalore, the time to show our
mettle has come. The last lap in scaling the Everest Peak is
the real tough one, when we cannot afford to make any mistake
and give our all to achieve what we have been working for so
long. We have moved so far above and the peak is almost there.
Can we afford not to scale the Peak?
Hum Kisi Se Kam Nahin!
Competing and meeting tough standards in vaccine and
pharmaceuticals is what distinguishes smart companies from
'also-rans'. Our Bangalore (Pharma) unit has faced more than
17 international regulatory audits and about 23 international
customer audits. Despite all this experience and rigour of
QMS, it was no easy task to satisfy USFDA auditor when she
came to the unit from 3rd to 7th September, 12.
Three months before this JMD had asked the 'Bangalore Pharma
team' whether they would like to have a US Consultant help
them with the preparation. The team went into introspection,
reflected on the offer and decided that they had enough
competence within to manage the USFDA audit without
International expert's help. It was no easy decision mind you.
All the same it will save hefty fees that would outflow at
this critical juncture.
Although the team had refused this help but inside they became
nervous when they heard that another reputed company in
Bangalore was taking similar help. What if they failed to
clear the audit? This spurred them into giving their more than
hundred percent effort. Their honor was at stake now.
Two weeks before the final audit, even a mock audit by
Oncology QA head was conducted to ensure that every action
would go as planned and there should be no oversight of any
kind. It was a relief to get no major observation in this mock
audit. Besides, minor observations were complied with
immediately.
"The five days of September from 3rd to 7th were full of ups
and downs like a 20-20 cricket match. Not a single ball could
be wasted", added Chirag Chopra, QA head. The USFDA auditor,
Dr. Ritu Malik was very thorough and dug deep into documents
whenever some doubt came in her mind. Only when all the
documents were tallying and answered the query correctly would
she be satisfied. Being an Indian doing an audit on behalf of
US government, there was greater rigour in her approach
auditing an Indian company. Her own reputation was also at
stake.
Finally, she cleared the audit with not even a single 483
observation. However she gave many useful suggestions for
further improvement of the systems. Moreover, she observed,
"Your system and plant is better in many respects than other
companies I have audited in this trip at Bangalore."
Congratulations to Team Bangalore (Pharma) led by Mr Palash
for this achievement. It has boosted the morale of entire PBL
family at this crucial juncture.
Now our Bangalore unit can market X & Ycapsules, AB
tablets to US. Smartly enough, the USFDA audit was also done
for Z for which ANDA was filed on 5th September while the
audit was in progress.
Our Netherland partner Ostimic can now go ahead full steam to
prepare for marketing of our products in the near future.
If winter comes, can spring be far behind? The flowers have
started blooming...
Behavioural Interventions to Unlock the Value Hidden in Pharma Companies: Lessons from Sun Pharma and Ranbaxy Deal
Ranbaxy was sold to Daiichi Sankyo by Singh brothers in 2008 at a price of Rs 737 per share*. Once again Daiichi has sold this company to Sun Pharma at Rs 457* per share on 8th April, 2014. If we take into account the inflation and loss of rupee value into account, it is a massive loss in value; more than two third of enterprise value lost in last six years! During the same time, Sun Pharma share value increased from approximately Rs120 ( face value of Rs5) to Rs 2900 (Rs580 face value of Rs 1 so actual value of Rs 2900)**. The value of Sun pharma increased more than 20 times (if we take inflation also into account) during the same period.
How this value was lost in case of Ranbaxy? Ranbaxy lost its credibility in the eyes of US FDA and was found to have many unethical practices including but not limited to destroying as well as fudging data for drug approvals. It resulted in total loss of US business from India because all the facilities suffered from import alert and could not sell anything to USA.
Ranbaxy had hired the best of technical consultants to manage the cGMP for these facilities, but to no avail. Reason is very simple yet profound. The causes of US FDA audit failures were behavioural and not technical as can be seen from the 483 issued to it for various facilities. Whereas solutions sought to be given were technical!! It lost value because it failed to manage the behaviour of frontline staff who run facilities; now Sun Pharma will have to provide the right nurturance and oversight in behavioural aspects to deal with these issues to unlock the hidden value.
It will not be incorrect to say that Ranbaxy lost more than 5 billion dollars in its value due to mismanagement of behavioural aspects of cGMP in last six years.
On the other hand Sun Pharma increased its value. What has been the secret of such tremendous growth in value?
In a stroke of genius, Sun Pharma led by Dilip Sanghvi, MD & CEO and Israel Makov, Chairman have utilized their capacity and potential to manage Pharma facilities and market to unlock the value hidden in the potential of Ranbaxy as of today. They have successfully integrated many other pharma facilties and markets through this inorganic growth path.
Can we say Ranbaxy value is not really lost but is locked and hidden only to be unleashed when it will be led by capable management?
Stroke of genius is ability to see this hidden and locked value close to 5 billion rupees in a distressed company!!
Israel Makov, who was Chairman of Teva, the largest Pharma company in the world based in Israel before joining Sun pharma, had done similar miracles in his old company. By doing such miracles he had increased the turnover of Teva from $2 billion to $8 billion in five years of his being the CEO. Of course it is not easy to increase turnover when it is already an astronomical number of $2 billion. Now listen to the story of Sun Pharma’s growth. It took Sanghvi 28 years to reach $1 billion; it took him less than 2 years to double it to $2 billion and now he is estimating that it will take him four years to reach $4 billion in sales with Israel to guide and mentor him. They have challenged the paradigm that once you become big you grow slower!
The real message hidden in above story: hire and manage well people who can increase value for you from various sources especially top level team members. This thesis is also validated in the way warren Buffet runs his Berkshire. He has hired the best CEOs to run his myriad acquisitions and has delegated completely his day to running to these best brains. You will find this secret operating in the most of wealth creating companies.
In sum, if you know how to get, retain and look after the best brains who will keep on increasing the value of your company, you are bound to grow and prosper as an entrepreneur! Mind you, the reverse is equally true. Mediocre people will keep on reducing the value of your company!! And it’s success lies in your people management competence.
*(422913803 shares of face value Rs 5 -Ranbaxy)
**(Sun Pharma share value 123 Rs on 3rd april, 2008 face value Rs 5 (*207116391)each and value of Rs 588 on 9th april, 2014 when it acquired Ranbaxy with face value of Rs 1 (*1035581955))
Values Hierarchy for GBP
Values are the basic foundation on which good behavioural practices for GMP are based. The most important value for any Pharmaceutical industry is integrity which will ensure that patients get safe and effective medicines. Under no circumstances patient safety can be compromised. Any act that violates this value is taboo (ethically worse than the thought or act of having incestuous relations).
Values always exist in the form of hierarchy. Whenever we have to make a choice, two or more values may come in conflict. For instance my value for non-violence and survival will come in conflict when somebody attacks me to kill. If my value of non-violence is higher in hierarchy than I will not attack my killer and would get killed rather get violent in self defense. On the other hand if my survival value is higher in the hierarchy, I will use violence in self defense even though non-violence is my important value.
In the GMP religion for Pharma industry, we advocate unequivocally that Patient safety value comes higher in hierarchy than the survival of the manufacturing company itself. If at any point the choice is between action to choose patient safety versus survival of the company, GMP person will choose patient safety; the company has to close down.
The base of any value is a very simple process. It was stated by Jesus 2000 years ago: Do not do unto others which you do not want others to do to you. You would not like to take a vaccine for your child which will be toxic for him/her. Would you like to produce a vaccine which may be toxic for your customers’ children?
Why it is difficult to follow such values? Naturally following values may entail suffering financial or other losses in the short term; while in the long term by earning trust, they will get the good will and business from customers. Unfortunately, most of us are caught in short term interests and choose to act unethically. It is only much later when the long term losses start facing us that we may realize our mistakes.
No wonder people deeply respect ethical persons because it is not for the chicken hearted to be ethical. It requires courage and wisdom to suffer losses in the short term.
No wonder again, it is difficult to find such ethical persons. Then how to ensure patient safety? For majority of the people the external stick will be required to pursue ethical choices. Hence top management has to put in place zero tolerance policy for ethical violations that may affect patient safety even remotely. The policy simply can be put in two sentences: First violation by any person attracts strong warning. On discovery of second violation the person is asked to leave the organization. Period. No discussions, no arguments on this.
Other elated facet is to have an Ethics Committee in place where anyone can lodge any complaint of ethical violations at any time. Based on inquiry and recommendations of the committee, swift and firm action by top management should follow without fail.
Another very important point, that we would like to highlight is a research finding from amongst the 5268 leaders in five different organizations. Put simply, it says that the integrity at the top will percolate down to lower levels. Second the level of integrity will slide with the levels as you move down, as shown in the graph below ( Source: HBR article by by Jack Zenger and Joseph Folkman ).So integrity starts at the top!
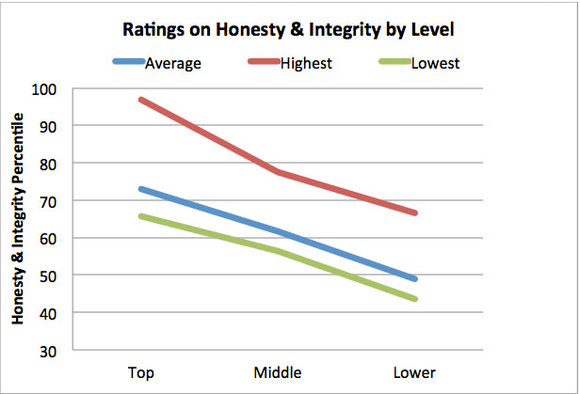
The conclusion is clear; we will have to have high integrity leaders at the top for GMP to flourish. No compromises on integrity at that level is warranted. Once that is ensured, half the battle is won; it will for sure percolate down to lower levels.
We have seen many cases of ethical violations in pharma industry which may compromise patient safety. Here is a sample list: 1. A purification step skipped to get higher yield of vaccine 2. No water sampling point existed but still daily water sampling report of the same point is being generated for years 3. Elisa tests on animals are showing positive while on papers it is documented negative ( all animals on which tests are to be conducted are shown healthy) 4. Fudged reporting of data e.g. differential pressure, temperature and humidity data which is to be recorded at periodical intervals etc 5. Fudged quality control test reports 6. Fudged validation studies prepared in office without actually doing the validation 7. Doing Non GMP batches in GMP facility 8. Showing training conducted on papers while on ground no training done 9. Operating facility even when differential pressure, temperatures and humidity out of range for hours or days 10. People with wounds going inside the GMP production facility for repairs or even for production 11. Back dated signatures is a common violation Etc
What are our expectations from GMP practitioners in terms of Value choices they ought to make for patient safety? Here is a list of do’s and don’t in terms of behaviour for their ready reference: 1. Refuse to obey the orders of your boss if actions based on that violate patient safety even remotely 2. Refuse to compromise on GMP procedures under management’s pressure such as to meet the deadlines, give higher yields etc 3. Write what you see and do what is written in SOPs etc 4. Lodge complaint to ethical committee in case you see any such violation happening at any place in the plant 5. A talisman you can use while practicing ethical behaviour is whether one can be transparent to others about what one is doing. Is it possible for you to reveal to the world about what you are doing or you need to hide it from others and are afraid of being found out?
What are the likely consequences for you? Will you lose your job? Would you like to work in an organization that does not care about patient safety? If it does not do so, tomorrow it will also not care about you, remember.
Will you feel pride in your work if you work for an unethical boss or organization? Do you want to belong to an ethical organization or dishonest organization?
Answer to above questions will give you enough courage to make the choices recommended above.
Further, to make things simpler, the incentives to be ethical can be listed thus: • One may earn one’s own respect and respect of others who value ethics; • One may get trust of people one deals with and therefore gain consequentially; • One may build stronger relationships based on high level of trust and respect; • One will have a clear conscience and relatively peaceful existence;
In the end I would like to give some ways in which we get out of our guilt in doing unethical actions. You may catch yourself doing the same and hence prevent using these defenses and thus follow the path of ethics. • Everybody is doing this ( say fudging reports) • I am doing it for company and not for myself ( creating fudged validation reports) • Only this one time • From tomorrow I will not do this • My friend comforting me by telling “You are right in doing this” • I am only obeying the orders of boss/ management • My company will not survive • I will lose job if I do not do this • They are making me do it • It has always been done like that • Nobody will object to this • I can easily get away doing this • No one is going to find out • I am not doing anything wrong ( even when others tell us so) • I am doing it because we do not have enough resources etc • They are doing it, I am not doing it • Who are you to ask me all these questions (aggression/ anger) • Trivialize it through humour and laughter • Yes. Yes, yes I am doing it and then continue doing it (quick acceptance) • In India so many percentage of companies/employees are doing it ( intellectualize it) • Let’s discuss weather rather than what you are asking • Withdraw and sulk on being questioned • Become extremely afraid and fearful along with submissiveness on being questioned about your behaviour.
In the end we would say that please do not choose a career in Pharmaceutical industry if you cannot follow the GMP religion.

Good Behavioural Practices
“At the heart of enduring success for any Pharmaceutical company lie Good Behavioural Practices- GBP”
Good Behavioural Practices - GBP emerged from our Behavioural Consulting with Pharmaceutical manufacturing Organizations. There is a business compulsion of implementing cGMP (Current Good Manufacturing Practices) in these companies if they have to get regulatory approvals for manufacturing and exporting medicines.

GBP Audit
It is well established in our experience of consulting with Pharma organizations that cGMP cannot be implemented without first establishing GBP. Establishing GBP requires extensive cultural transformations.
GBP Audit is a proprietary tool designed by UTSAV to enable Pharma organizations understand their readiness and maturity in implementing cGMP on enduring basis.
Read More

Distance Learning Program for GBP
With distance learning programme UTSAV aim to educate the Pharmaceutical executives at senior levels( with minimum ten years industry experience) on behavioural knowledge, skills and attitude necessary to implement GMP and QMS.
At the successful completion of programme, requiring written test and viva voce, the participants will be awarded certificate of completion of First Level GBP programme.
Case Studies
Read case studies of UTSAV clients who used our Good Behavioural Training Programs to drive their growth and build their brands.
A case study is a comprehensive study of a social unit of society, which may be a person, family group, institution, community or event. A case study focuses attention on a single unit thoroughly. The aim is that to find out the influencing factors of a social unit and the relationship between these factors and a social unit.

HR Communication & Newsletters
GBP based communication is a powerful tool to bring about change towards GBP. And it is an art few know how to implement in organization. We have developed a special expertise in the same and many clients have used it to their immense benefit. Please find below the actual samples of communication designed by us for the CEO of a Pharmaceutical orhganization.
Read More

Blog
Good Behavioural Practices can ensure competitive success for Indian Pharmaceutical industry. However, Indian Industrial culture is still to fully come out of ‘Chalta Hai’ mindset. Everyone knows that large numbers of Pharmaceutical plants need vast improvement in the work culture to compete in the world markets.
Read More

Pharma - Behavioural Issues
Pharmaceutical companies face complex issues that grow more challenging by the day
One of the most crucial questions facing the industry, though, is what leadership skills companies will need to navigate this complex and changing landscape—and how current pharmaceutical leaders stack up.
Read More

Video
"When you educate a man, you liberate a man." – Dr. Benjamin Carson.
Dr. Carson, a distinguished man of science and healing, the Director of the Pediatric Neurosurgery Division at the John Hopkins Hospital in Baltimore, talks about political correctness and how it can muzzle an entire nation. What is the point of an education if we do not voice our opinions on important issues?
Read More

 +91 9810009311
+91 9810009311 pradeepprakash2001@gmail.com
pradeepprakash2001@gmail.com
